The bond angle in a trigonal pyramidal molecule is approximately 107 degrees. This angle determines the overall shape and properties of the molecule, influencing its reactivity and behavior. Understanding the trigonal pyramidal bond angle is crucial in predicting molecular geometry and interactions. By delving into this fundamental concept, we can unravel the intricacies of molecular structures and their significance in chemistry. Let’s explore the fascinating world of trigonal pyramidal bond angles and uncover the secrets they hold within chemical bonds.
Understanding Trigonal Pyramidal Bond Angles: A Simple Guide
Welcome to our exploration of the fascinating world of trigonal pyramidal bond angles! In this blog post, we will delve into the concept of trigonal pyramidal molecules, their unique structure, and the bond angles that define their geometry. So, let’s embark on this exciting journey together!
What are Trigonal Pyramidal Molecules?
Trigonal pyramidal molecules are a type of molecular geometry that is commonly observed in chemical compounds. These molecules have a central atom surrounded by three bonding pairs and one non-bonding pair of electrons, resulting in a pyramid-like shape.
One of the most well-known examples of a trigonal pyramidal molecule is ammonia (NH3). In ammonia, the central nitrogen atom is bonded to three hydrogen atoms and has one lone pair of electrons, giving it a trigonal pyramidal shape.
The Role of Bond Angles in Trigonal Pyramidal Molecules
Bond angles play a crucial role in determining the overall shape of a molecule. In trigonal pyramidal molecules, the bond angles are the angles between the central atom and the surrounding atoms. These angles are essential in defining the three-dimensional structure of the molecule.
Understanding the Ideal Bond Angle
The ideal bond angle in a trigonal pyramidal molecule is approximately 107 degrees. This angle is a result of the repulsion between the bonding pairs and the lone pair of electrons around the central atom.
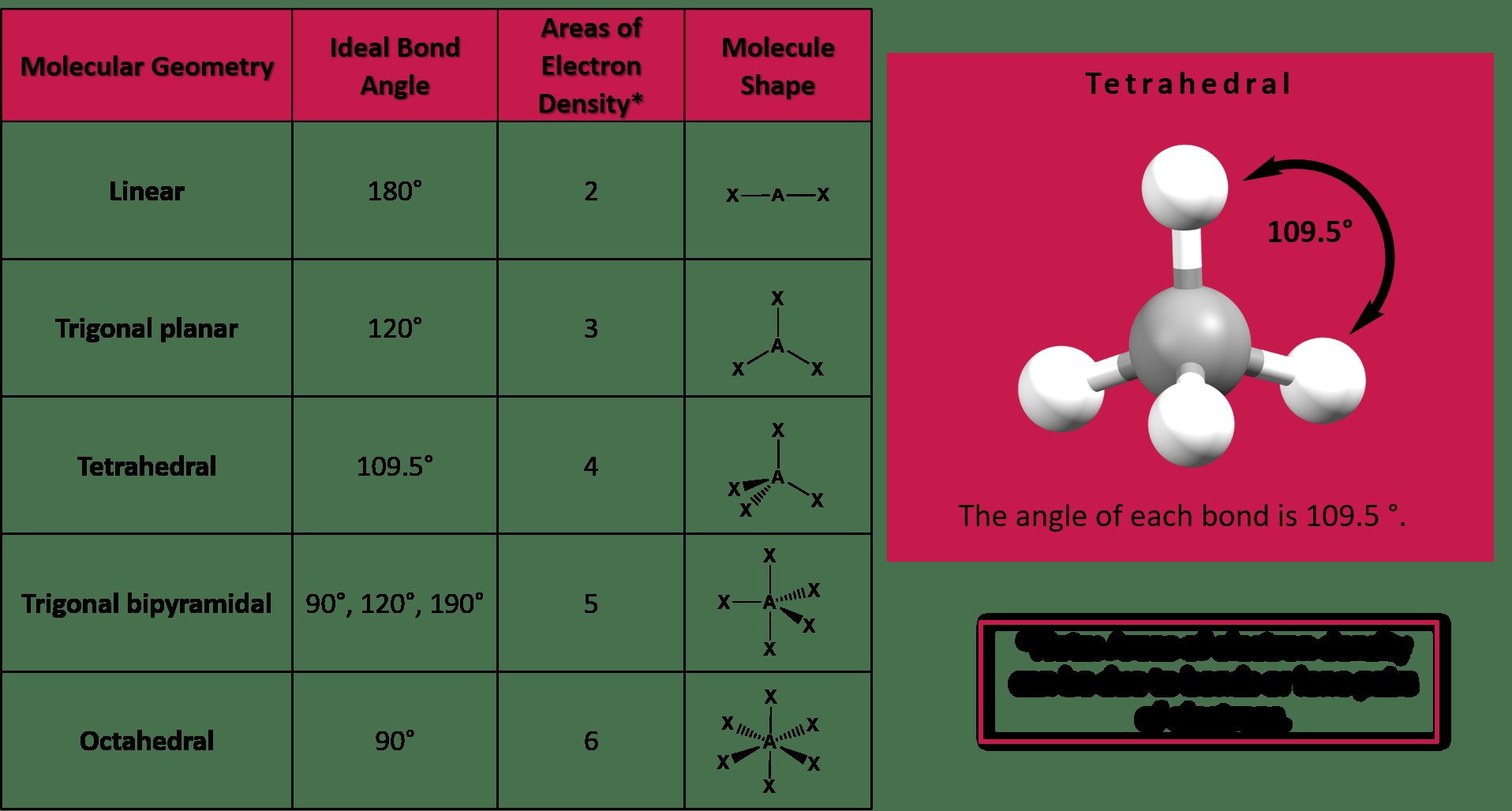
When we visualize a trigonal pyramidal molecule like ammonia, the three hydrogen atoms and the lone pair of electrons repel each other, causing the bond angles to be slightly less than the ideal 109.5 degrees found in a perfect tetrahedral geometry.
Factors Affecting Trigonal Pyramidal Bond Angles
Several factors can influence the bond angles in trigonal pyramidal molecules. Let’s explore some of the key factors that affect the bond angles:
Presence of Lone Pairs
The presence of lone pairs of electrons on the central atom can affect the bond angles in a trigonal pyramidal molecule. Lone pairs tend to exert greater repulsion compared to bonding pairs, leading to a distortion in the bond angles.
For example, in ammonia, the lone pair of electrons occupies more space than a bonding pair, resulting in a slightly smaller bond angle than the ideal 109.5 degrees.
Electronegativity of Atoms
The electronegativity of the atoms in a trigonal pyramidal molecule can also impact the bond angles. Atoms with higher electronegativity tend to pull electron density towards themselves, affecting the distribution of electrons and, consequently, the bond angles.
For instance, in a molecule like hydrogen fluoride (HF), the fluorine atom’s high electronegativity causes the bond angle to deviate slightly from the expected 107 degrees in a trigonal pyramidal geometry.
Steric Effects
Steric effects, which refer to the repulsion between electron pairs or atoms, can influence the bond angles in trigonal pyramidal molecules. When bulky groups are present around the central atom, steric hindrance can alter the bond angles and distort the molecular geometry.
For example, in a molecule like phosphine (PH3), the presence of bulkier phosphorus atoms can lead to deviations in the expected bond angles due to steric interactions.
Real-Life Applications of Trigonal Pyramidal Bond Angles
Trigonal pyramidal molecules and their bond angles have several practical implications in the field of chemistry and beyond. Let’s take a look at some real-life applications of trigonal pyramidal bond angles:
Biological Molecules
In biological systems, molecules with trigonal pyramidal geometry play essential roles. For instance, the structure of water molecules (H2O) exhibits a bent shape with bond angles of approximately 104.5 degrees, reflecting its trigonal pyramidal geometry.
The unique bond angles in water molecules are crucial for its properties, such as polarity, hydrogen bonding, and solubility, which are fundamental in various biological processes.
Chemical Reactivity
The bond angles in trigonal pyramidal molecules influence their chemical reactivity and properties. Understanding the geometry and bond angles of molecules helps chemists predict how they will interact with other substances, participate in reactions, and form new compounds.
For example, the bond angles in ammonia determine its ability to act as a Lewis base and form complexes with metal ions, showcasing the significance of trigonal pyramidal geometry in chemical reactivity.
Molecular Shape Determination
Trigonal pyramidal bond angles are crucial for determining the overall shape and properties of various molecules. By analyzing the bond angles in a molecule, chemists can predict its behavior, physical properties, and interactions with other substances.
This knowledge is instrumental in fields such as drug design, material science, and environmental chemistry, where understanding molecular geometry is essential for developing new compounds and technologies.
In conclusion, trigonal pyramidal bond angles are a fundamental aspect of molecular geometry that play a significant role in determining the shape, properties, and reactivity of molecules. By understanding the factors that influence bond angles in trigonal pyramidal molecules, we can unravel the mysteries of chemical structures and their real-world applications.
We hope this guide has provided you with valuable insights into the fascinating world of trigonal pyramidal bond angles. Stay curious, keep exploring, and remember that chemistry is all around us!
Trigonal Pyramidal Molecular Geometry/Shape and Bond Angles
Frequently Asked Questions
What is the bond angle in a trigonal pyramidal molecule?
In a trigonal pyramidal molecule, the bond angle is approximately 107 degrees. This bond angle arises from the arrangement of three bonding pairs and one lone pair of electrons around the central atom, creating a tetrahedral geometry that is slightly distorted due to the lone pair.
How does the presence of a lone pair affect the bond angle in a trigonal pyramidal molecule?
The presence of a lone pair in a trigonal pyramidal molecule repels the bonding pairs more strongly than if the molecule had no lone pairs. This lone pair repulsion causes the bonding angle to be slightly less than the ideal tetrahedral angle of 109.5 degrees, resulting in a bond angle of around 107 degrees.
Why is the bond angle in a trigonal pyramidal molecule less than the tetrahedral angle?
The bond angle in a trigonal pyramidal molecule is less than the ideal tetrahedral angle of 109.5 degrees due to the repulsion between bonding and lone pairs of electrons. The lone pair occupies more space around the central atom, pushing the bonding pairs closer together and reducing the bond angle to around 107 degrees.
Final Thoughts
In conclusion, the trigonal pyramidal bond angle is a key characteristic of molecules with this molecular geometry. Understanding this bond angle is crucial for predicting the overall shape and properties of the molecule. By knowing the trigonal pyramidal bond angle, scientists can better comprehend the molecular behavior and interactions that occur within these compounds. Mastering this concept enhances our knowledge of chemistry and provides valuable insights into the structural arrangements of various molecules.